COMPUTER SIMULATIONS OF EPOXY BINDING ON IRON OXIDE SURFACES - poster
Charlie Wand1, Simon Gibbon2 and Flor Siperstein1
1 Department of Chemical Engineering and Analytical Science, The University of Manchester, Oxford Road, Manchester, M13 9PL, UK
2 AkzoNobel Research & Development, Northallerton, North Yorkshire, DL7 7BJ, UK
Epoxy resins are widely used in protective coatings due to their good heat and chemical resistance, favourable mechanical properties and good adhesion to a range of substrates. As such, epoxy resins have been formulated as a protective coating for a wide range of applications, from aerospace and marine applications through to nontoxic interior coatings in the food industry [1]. In all cases, the performance of the final solid-polymer system is dependent on the physicochemical properties of the interface and the interaction between the polymer and the solid substrate. However, experimental methods to characterize this interaction are limited and mostly deteriorative to the interface. Computer modelling provides a tool to investigate the surface-polymer interface at an atomistic level.
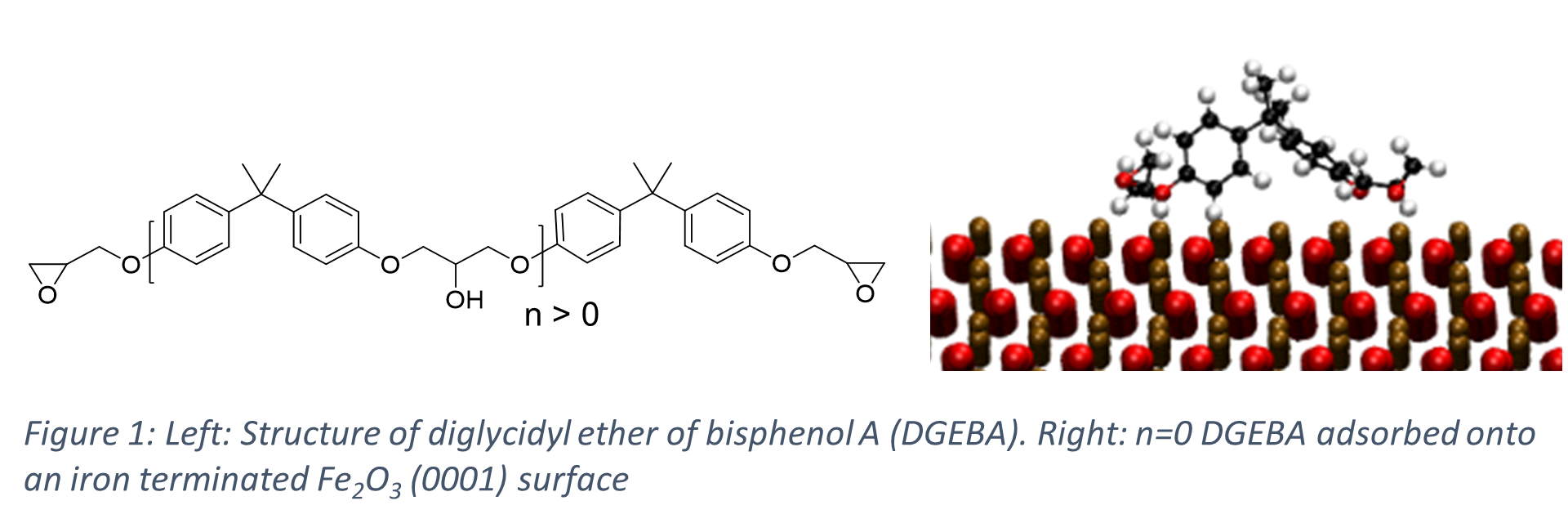
Here we perform atomistic molecular dynamics simulations to investigate the binding of a common component in epoxy resins, diglycidyl ether of bisphenol A (DGEBA), on Iron Oxide surfaces (Figure 1) and investigate the effect of number of repeat units in DGEBA on the binding energy (ΔEbinding) defined as;
ΔEbinding= Eadsorbate/surface-(Eadsorbate+Esurface )
Where Eadsorbate/surface is the energy of the adsorbed DGEBA on the surface and Eadsorbate, Esurface are the energy of the adsorbate and surface in vacuum respectively. In epoxy resin applications the composition of the solid substrate is highly varied, with pre-treatments and production processes leading to a non-uniform surface chemistry and roughness. To reflect this, we investigate two Iron Oxides surfaces, hematite (Fe2O3) and magnetite (Fe3O4). We find that binding is stronger for DGEBA on hematite than magnetite, in agreement with previous literature findings [2] and suggest causes of this trend based on the surface termination.
This work was done with support from the EPSRC Prosperity Partnership SusCORD (EP/S004963/1).
[1] Friedrich, Jörg. Metal-Polymer Systems: Interface Design and Chemical Bonding. John Wiley & Sons, 2017.
[2] Bahlakeh, Ghasem, et al J. Phys. Chem. C 120 20 (2016): 11014-11026.

